Cooperative ligands
In contrary to normal “innocent” ligands for transition-metal catalysis, a series of ligands that actively participate in the bond forming or breaking events during metal catalysis are regarded as “cooperative” or “non-innocent” ligands. In general, such ligand category involves ligands with an internal base to promote proton abstraction from the substrate, ligands for outersphere hydrogenation, and redox-active ligands.
Our research group has been focusing on two types of cooperative ligands – the hybrid olefin ligands for Pd/olefin cooperative catalysis, and the sulfoxide-2-pyridone ligands (SOHP) for C-H functionalization.
Hybrid olefin ligands
Olefin ligands have been employed as useful stirring ligands in transition metal catalysis, and the olefin unit always acts as an innocent ligand in the reaction. Inspired by the Catellani reaction, which features a Pd/norbornene (Pd/NBE) cooperative catalytic system, we designed a new type of hybrid olefin ligand that consists of a cycloolefin, a heteroatom coordination unit, and a tether to achieve a novel Pd/olefin cooperative catalysis.
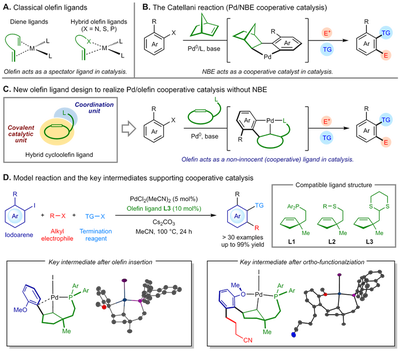
This work demonstrated for the first time that the Catellani-type reaction could be realized without using an NBE-related co-catalyst, which changed the long-standing cognition of the field. Key organometallic intermediates have been isolated and characterized to support the cooperative nature of the olefin ligand. Under the concept of Pd/olefin cooperative catalysis, more new reactivity is anticipated, and we hope this work to initiate the development of a more general transition metal/olefin cooperative catalysis in the future.
SOHP ligands
Regio- and stereo-selective direct C-H functionalization reaction of organic molecules has long been a formidable challenge in organic synthesis. In most cases C-H metalation determines the regioselectivity of the overall reaction, and the following functionalization step simply inherit this regio-preference. With an idea to make the C-H metalation step no longer rate-limiting by ligand-acceleration, we hypothesized that the functionalization step might play an important role in dictating the regioselectivity of the reaction, which could represent a new mode of regiocontrol in the C-H functionalization reaction.
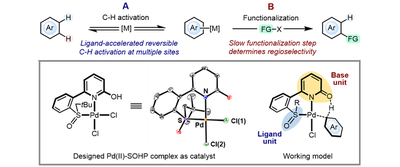
We designed a cooperative ligand bearing a 2-pyridone (2-hydroxypyridine) base unit and a sulfoxide ligand unit, hoping to accelerate the C-H activation step in palladium catalysis. With the oxidative Heck reaction of indole as a model, we found that this sulfoxide-2-hydorxypyridine (SOHP) ligand significantly accelerated the C-H activation on the indole core, shifting the rate-limiting step to the subsequent alkene insertion. Based on this design, we have achieved the ligand-controlled regioselectivity in this reaction. This ligand also exhibits potential on the regio- and stereo-control of various types of C-H functionalization reactions.
Major contributors
Key references
- Zheng, Y.-X.; Jiao, L.* “Hybrid cycloolefin ligands for palladium/olefin cooperative catalysis.” Nature Synthesis 2022, 1, 180-187.
- Wang, F.-Y.; Jiao, L.* “Functionalized cycloolefin ligand as a solution to ortho-constraint in the Catellani-type reaction.” Journal of the American Chemical Society 2023, 145, 4871-4881.
- Wang, Y.-J.; Yuan, C.-H.; Jiao, L.* “Regiocontrol in the oxidative Heck reaction of indole by ligand-enabled switch of the regioselectivity-determining step.” Chemical Science 2020, 11, 11042-11054.
- Yuan, C.-H.; Wang, X.-X.; Jiao, L.* “Ligand-enabled Pd(II)-catalyzed enantioselective β-C(sp3)-H arylation of aliphatic tertiary amides.” Angewandte Chemie International Edition 2023, 62, e202300854.