Alkaloid synthesis
Natural product synthesis belongs to one of our longstanding research interests. Since 2014 we have worked on the total synthesis of several monoterpene indole alkaloids based on the development of new strategies, including minfiensine, vincorine, strictamine, and arborisidine.
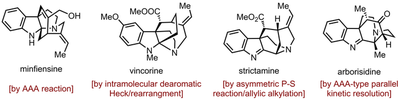
We have also devoted in the development of new synthetic methodologies for the construction of indole skeletons with quaternary stereocenters, which are potentially useful in indole alkaloid synthesis.
Major contributors
Key references
- Zhang, Ze-Xin; Chen, Si-Cong; Jiao, Lei* “Total synthesis of (+)-minfiensine: construction of the tetracyclic core structure by an asymmetric cascade cyclization.” Angewandte Chemie International Edition, 2016, 55, 8090-8094.
- Gao, Dong; Jiao, Lei* “Construction of indoline/indolenine ring systems by a palladium-catalyzed intramolecular dearomative Heck reaction and the subsequent aza-semipinacol rearrangement.” The Journal of Organic Chemistry, 2021, 86, 5727-5743.
- Wang, Feng-Yuan; Jiao, Lei* “Total synthesis of (-)-arborisidine.” Angewandte Chemie International Edition, 2021, 60, 12732-12736.