BHAS reaction mechanism
The base-promoted homolytic aromatic substitution (BHAS) reaction had been a topic of great interest since 2010. However, in spite of the synthetic advances, the key question regarding the radical initiation mechanism remained debatable and unsolved. We focused on a representative diamine-promoted BHAS reaction system, and our detailed mechanistic studies have provided useful mechanistic insights.
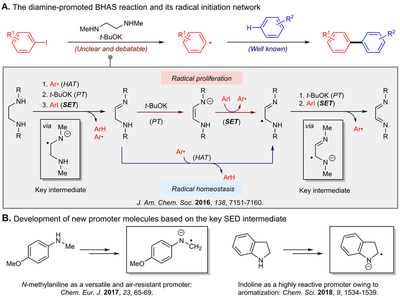
This work identified imino radical anions as the key reactive intermediates in diamine-promoted BHAS reaction and elucidated a complex mechanistic network that controls the aryl radical concentration, which contrasts the previously proposed mechanistic model. Based on the reactivity of the key radical anions, two novel amine-type small molecule promoters for the BHAS reaction with greater reactivity have been figured out by structural modification, demonstrating the importance of mechanistic understanding.
Major contributors
Key references
- Zhang, Li; Yang, Huan.; Jiao, Lei* “Revisiting the radical initiation mechanism of the diamine-promoted transition-metal-free cross-coupling reaction.” J. Am. Chem. Soc. 2016, 138, 7151-7160.
- Yang, Huan; Zhang, Li; Jiao, Lei* “N-Methylanilines as simple and efficient promoters for radical-type cross-coupling reactions of aryl iodides.” Chem. Eur. J. 2017, 23, 65-69.
- Yang, Huan; Chu, De-Zhao; Jiao, Lei* “Aromatization modulates the activity of small organic molecules as promoters for carbon–halogen bond activation”, Chem. Sci. 2018, 9, 1534-1539.