Abstract
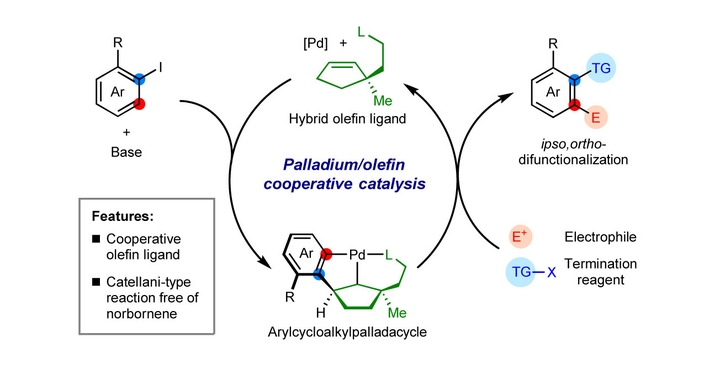
Engineering of molecular catalysts in homogeneous catalysis enables new catalytic modes, leading to efficient synthetic transformations. In this regard, design of ligands for transition-metal catalysis has played a major role. In transition metal catalyzed reactions, olefins can serve as steering ligands by tuning the electronic and steric nature of the metal centre. Here we report unstrained olefin ligands bearing a P or S coordination site for use in the Pd-catalyzed Catellani reaction. This olefin shows covalent catalytic function and enables efficient ipso,ortho-difunctionalization of iodoarenes. Mechanistic analysis reveals reversible covalent bonding between the substrate and the cycloolefin unit of the ligand, forming key organopalladium intermediates to enable new reactivity. Our results demonstrate a design concept that employs hybrid cycloolefin ligands as a covalent catalytic module, opening possibilities for further cooperative catalysis with other transition metal–olefin hybrids.
Note: This article was posted on ChemRxiv as a preprint before its publication. Please visit DOI:10.33774/chemrxiv-2021-9mgk2-v2 for details.
Highlights
- Highlighted in Synfacts 2022, 18, 503.
- Promoted by 分子魔术师 on WeChat.