Abstract
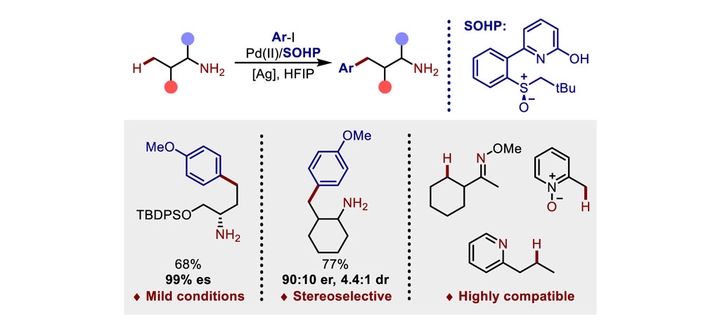
Amines are common scaffolds in bioactive molecules as well as building blocks for chemical synthesis. Despite significant advances in palladium-catalyzed C(sp3)−H functionalization of amines over the past decades, it remains challenging to perform directed C−H metalation with native amine groups rather than amine-derived or transient directing groups (DGs). Recently, our group developed the Pd(II)/sulfoxide-2-hydroxypyridine (SOHP) catalytic system, in which SOHP ligands play a pivotal role as a crucial functional module, enabling regioselective C(sp2)−H and enantioselective C(sp3)−H functionalization reactions. In this work, we demonstrate that this chemistry provides an ideal solution for native primary amine-directed γ-C(sp3)-H arylation. Primary amines of varying degrees of complexity, encompassing amino acid esters and aminol silyl ethers, were found to be compatible with the established methodology. Additionally, we achieved a preliminary implementation of the asymmetric variant of this γ-C−H arylation reaction by employing a chiral SOHP ligand. Moreover, the range of applicable substrates could be extended to pyridine, oxime ether, and pyridine-N-oxide.
Note: This article was posted on ChemRxiv as a preprint before its publication. Please visit DOI:10.26434/chemrxiv-2023-dzrp1 for details.