An Umpolung Approach to the Hydroboration of Pyridines: A Novel and Efficient Synthesis of N-H 1,4-Dihydropyridines.
Abstract
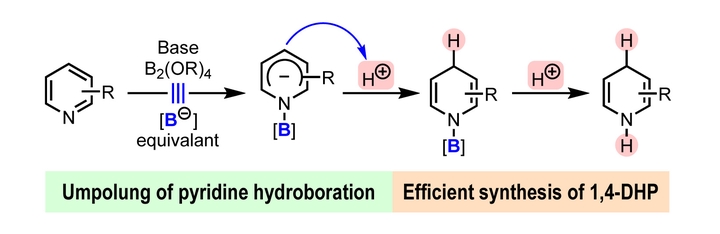
Umpolung of pyridine hydroboration was achieved by the reaction between pyridine and diboron(4) with a base and a proton source. , The first inverse hydroboration of pyridine with a diboron(4) compound and a proton source has been realized under simple basic and catalyst-free conditions. This process consists of a formal boryl anion addition to pyridine, which produces an N -boryl pyridyl anion complex, and the subsequent protonation of the anion complex. This process enables a simple and efficient method for the synthesis of multi-substituted N -H 1,4-dihydropyridine (1,4-DHP) derivatives that are difficult to prepare using established methods. Furthermore, this method allows for facile preparation of 4-deuterated 1,4-DHPs from an easily accessible deuterium ion source. This inverse hydroboration reaction represents a new mode for pyridine functionalization.