N-Methylanilines as Simple and Efficient Promoters for Radical-Type Cross-Coupling Reactions of Aryl Iodides.
Abstract
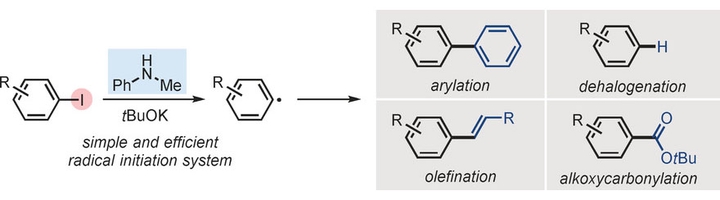
Activation of the carbon– halogen bonds in aryl halides is a key step in transition-metal-free cross-coupling reactions. In this paper, a new and efficient radical initiation system for the activation of iodoarenes to produce aryl radicals was discovered, which employs the combination of N-methylanilines and tBuOK. This radical initiation system is robust and versatile, enabling various types of aryl-radical-related reactions.
Publication
Chem. – Eur. J. 2017, 23, 65-69.
Highlights
- Highlighted in Synfacts 2017, 13, 191.