Abstract
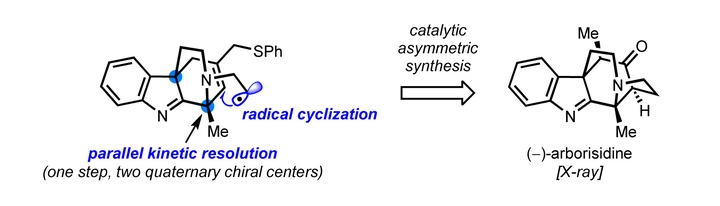
An asymmetric total synthesis of cage-like indole alkaloid arborisidine is presented. The new synthetic strategy features a catalytic parallel kinetic resolution based on ambident nucleophilicity (C3/N) of indole to set the absolute configurations of the two quaternary chiral centers, and a 5-exo-trig radical cyclization to form the bridged nitrogen-containing five-membered ring.
Publication
Angew. Chem. Int. Ed. 2021, 60, 12732-12736.