Divergent Synthesis of Indolenine and Indoline Ring Systems by Palladium-Catalyzed Asymmetric Dearomatization of Indoles.
Abstract
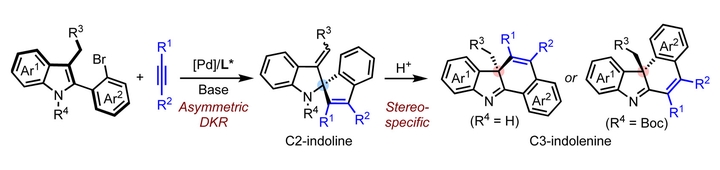
Dearomatized indole derivatives bearing a C3- or C2-stereocenter exist ubiquitously in natural products and biologically active molecules. Despite remarkable advances in their chemical synthesis, stereoselective and regio-divergent methods are still in a high demand. Herein, a Pd-catalyzed intermolecular asymmetric spiroannulation of 2,3-disubstituted indoles with internal alkynes has been developed for the efficient construction of indoline structures with a C2-quaternary stereocenter. Stereospecific aza-semipinacol rearrangement of these indoline derivatives under acidic conditions afforded indolenine products bearing a C3-quaternary stereocenter, where the selectivity for the rearranging group could be controlled by the reaction sequence. The asymmetric spiroannulation together with the subsequent aza-semipinacol rearrangement enabled a divergent access to dearomatized indole derivatives with either a C3- or a C2-quaternary stereocenter.
Note: This article was posted on ChemRxiv as a preprint before its publication. Please visit DOI:10.26434/chemrxiv-2021-t61fx-v2 for details.