Chiral Ligands for Palladium-Catalyzed Coordination-Assisted Enantioselective C(sp3)-H Functionalization Reactions (Invited Review).
手性配体在钯催化配位辅助对映选择性C(sp3)-H键官能团化反应中的应用(中文综述)
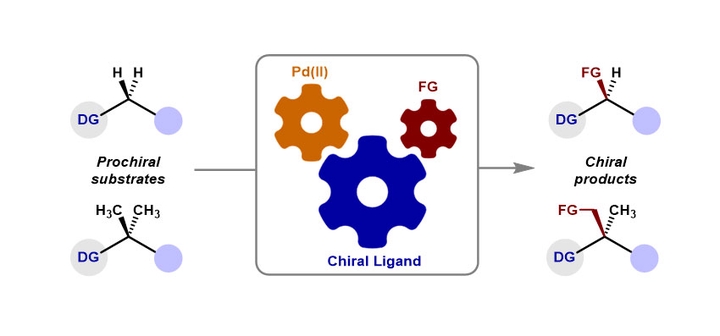
Abstract
Enantioselective C(sp3)-H functionalization reactions have emerged as a powerful and straightforward strategy in the construction of chiral molecules. Palladium, highlighted by its remarkable reactivity, versatility, and functional group tolerance, stands out among transition metal catalysts and is one of the most prevalently employed in enantioselective C(sp3)-H functionalization reactions. In these reactions, chiral ligands play a pivotal role, serving as a decisive factor in controlling both reactivity and stereoselectivity. This review delves into advancements concerning palladium-catalyzed coordination-assisted enantioselective C(sp3)-H functionalization reactions, focusing particularly on the design principles, reaction mechanisms, and stereocontrol models of chiral ligands.