Thio-Cycloolefin Ligand Enabled Distal Methylation in the Catellani-Type Reaction via a Six-Membered Palladacycle.
Abstract
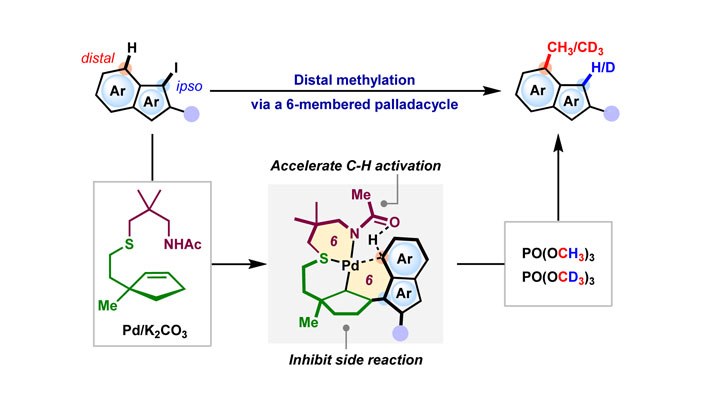
TThe Catellani reaction, also known as the Pd/norbornene catalysis, is a potent method for synthesizing multisubstituted arenes through the ortho-functionalization/ipso-termination process of haloarenes. This chemistry relies heavily on the formation of a five-membered palladacycle, restricting the ortho-functionalization to the aryl ring bearing the ipso-halogen. In this study, we present an innovative strategy to circumvent this limitation, expanding the synthetic capabilities of the Catellani-type reaction. By employing the Pd/olefin ligand cooperative catalysis developed within our research group, we successfully executed a non-classical Catellani-type reaction on a 6,5-fused heteroaromatic system via a six-membered palladacycle intermediate. The use of a functionalized thio-cycloolefin ligand with an amide group as the functional moiety and a connecting unit bearing a geminal-dimethyl group enabled distal C−H methylation at the 4-positions of 3-iodoindole, 7-azaindole, and indolizine rings, providing a novel pathway for the synthesis of methylated analogs of pharmaceutical compounds based on these scaffolds. This study highlights the potential of Pd/olefin ligand catalysis in unveiling new reactivity for advancing non-classical Catellani-type reactions.