Abstract
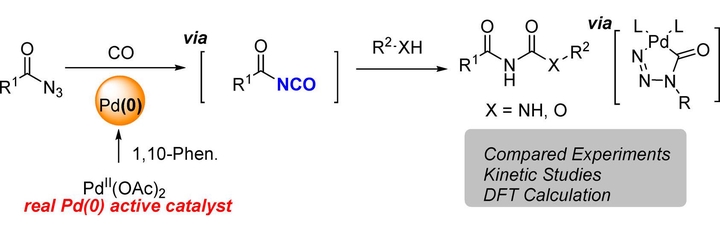
Pd-catalyzed reactions of azides with CO to access an isocynate intermediate have been developed extensively in recent years. However, the catalytic carbonylation of sensitive acyl azides has not been reported. Herein, we report a simple Pd-catalyzed carbonylation reaction of acyl azides with broad substrate scope, high efficiency, and simple operation under mild conditions, which provides facile access to acyl ureas. In addition, a mechanistic study was carried out by both experiment and DFT calculation. Control experiments and kinetic study revealed that the real active palladium species were Pd(0). The result of kinetic study suggested that palladium catalyst, azide, and CO were all involved in the turnover-limiting step except for amine. Further DFT study suggested that an unprecedented five-membered palladacycle intermediate was the key intermediate in the carbonylation reaction.