Asymmetric Defluoroallylation of 4-Trifluoromethylpyridines Enabled by Umpolung C-F Bond Activation.
Abstract
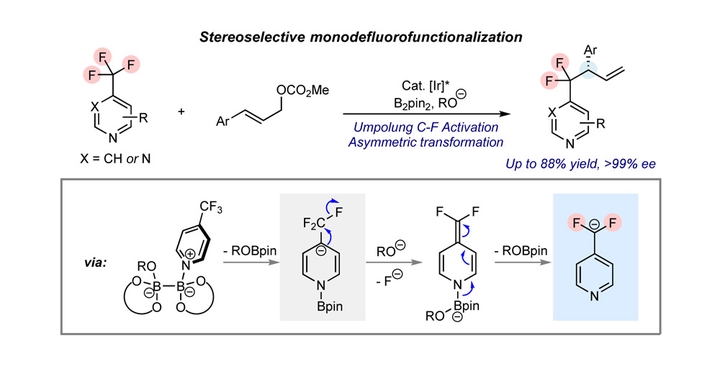
Carbon-fluorine bond activation reaction of the trifluoromethyl group represent an important approach to fluorine-containing molecules. While selective defluorofunctionalization reactions of CF3-containing substrates have been achieved by invoking difluorocarbocation, difluorocarboradical, or difluoroorganometallic species as the key intermedi-ate, the transformations via fluorocarbanion mechanism remained a limited success. Furthermore, the enantioselective defluorotransformation of CF3 group has not yet been realized. Herein, we report a defluorofunctionalization reaction of 4-trifluoromethylpyridines involving pyridyldifluoromethyl anion as the key intermediate, which was developed based upon our previous studies on the N-boryl pyridyl anion chemistry. When combined with Ir-catalysis, asymmetric defluoroallylation of 4-trifluoromethylpyridines could be achieved to forge a difluoroalkyl-substituted chiral center. The present work opens up a new opportunity for the defluorofunctionalization of CF3 group, and provides new insights into the N-boryl pyridyl anion chemistry.
Note: This article was posted on ChemRxiv as a preprint before its publication. Please visit DOI:10.26434/chemrxiv-2022-kk0ww for details.