Abstract
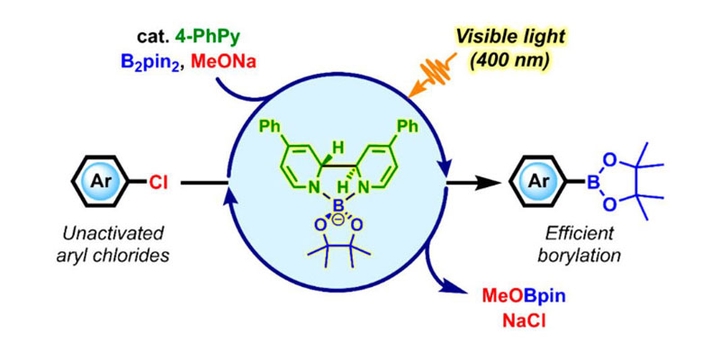
The preparation of arylboronates from unactivated aryl chlorides in a transition-metal-free manner is rather challenging. There are only few examples to achieve this goal by using ultraviolet irradiation. Based on the mechanistic understanding of the diboron/ methoxide/pyridine reaction system, we achieved photoactivation of the in situ generated super electron donor and developed a visible-light-induced organocatalytic method for efficient borylation of unactivated aryl chlorides.
Publication
J. Am. Chem. Soc. 2019, 141, 9124-9128.
Highlights
- Highlighted in Synfacts 2019, 15, 1053.
- Highlighted by SCIENTIFIC UPDATE with the title “Boron sees the light - A visible light induced organocatalytic borylation of aryl chlorides”.
- Included as an Abstract in the Organic Chemistry Portal.