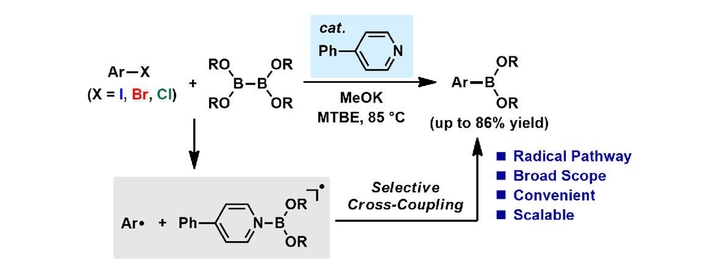
Abstract
A pyridine-catalyzed transition-metal-free borylation reaction of haloarenes has been developed based on the selective cross-coupling of an aryl radical and a pyridine-stabilized boryl radical. Arylboronates were produced from haloarenes under mild conditions. This borylation reaction features a broad substrate scope, operational simplicity, and gram-scale synthetic ability.
Publication
J. Am. Chem. Soc. 2017, 139, 607-610.
Highlights
- Highlighted in Synform 2017/04, A65-A66.
- Included as an Abstract in Organic Chemistry Portal.