Asymmetric Total Synthesis of (+)-Minfiensine by an Asymmetric Cascade Cyclization Strategy.
Abstract
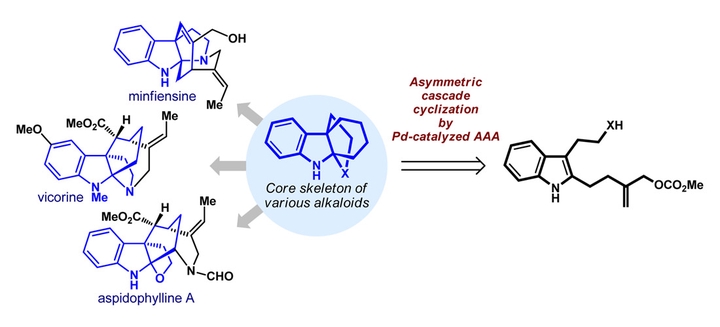
The Strychnos alkaloid minfiensine and a series of akuammiline alkaloids, such as vincorine, aspidophylline A, and picrinine, possess a common core skeleton, a 4a,9a-heterocycle-fused tetrahydrocarbazole. Efficient construction of this core structure in a highly enantioselective manner would facilitate the total synthesis of these alkaloids. In this article, we briefly summarize the established strategies for obtaining this core structure, together with the corresponding total-synthesis routes, and we describe our own effort on the development of a new strategy, the asymmetric cascade dearomative cyclization, for the efficient total synthesis of (+)-minfiensine.
Publication
Synlett 2017, 28, 2199-2204.