Photoinduced Radical Borylation of Alkyl Chlorides.
Abstract
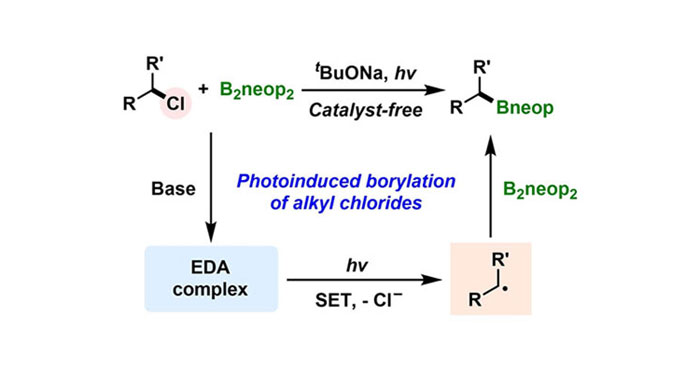
The traditional method for the preparation of alkylboronic esters involves transition metal catalysis or transition metal-free methods relying on single electron transfer. Each method relies on the suitable choice of catalyst or electron transfer mediator. In this study, we have developed a new method for the synthesis of alkylboronic esters from alkyl chlorides and diboron(4) compounds, without the need for additional metal or non-metal catalysts. We discovered that under 400 nm visible light irradiation, alkyl chlorides can be activated in the presence of bis(neopentyl glycolato)diboron and an alkoxide base to form alkyl radical intermediates, which then react with diboron(4) compounds, resulting in high yields of alkylboronic esters. The reaction is applicable to both primary and secondary alkyl chlorides. The simplicity of operation and the broad substrate scope of this method make it practical for the synthesis of valuable alkylboronic ester intermediates.