Palladium-Catalyzed ortho-Alkylation of Iodoarenes Enabled by a Cooperative Cycloolefin Ligand and a Bulky Trialkylphosphine (Synpacts).
Abstract
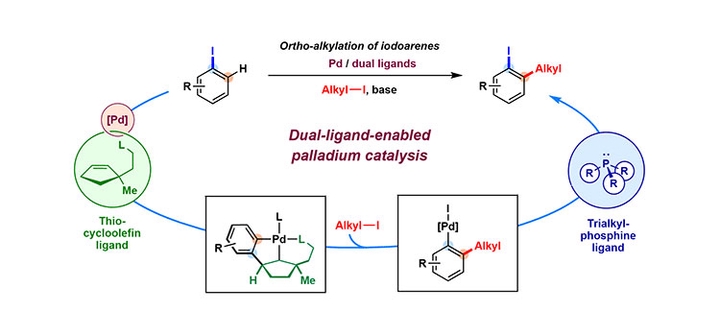
We recently achieved ortho-alkylation of iodoarenes utilizing a dual-ligand catalytic system that effectively combines palladium/olefin ligand cooperative catalysis with reversible C(sp2)–I reductive elimination enabled by a bulky trialkylphosphine ligand. Through careful mechanistic investigations, we confirmed the compatibility of the crucial steps involved in this process by isolating key organometallic intermediates and studying the stoichiometry of their transformations. By utilizing this protocol, we successfully achieved the ortho-alkylation of a variety of iodoarene substrates, thereby expanding the scope of applications for Catellani-type reactions. This study showcases the synthetic potential of the Pd/olefin ligand cooperative system as an innovative complement to established Pd/norbornene catalysis. In this Synpacts article, we present the conceptual framework underpinning this reaction and detail the key aspects of its development.