Nitrogen-Centered Radical Cyclization Initiated by Proton-Coupled Electron Transfer: A Mechanistic Study of 5-Exo-trig and 6-Endo-trig Cyclization Pathways.
Abstract
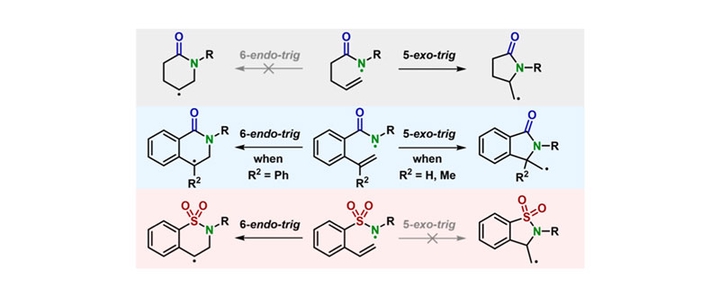
Nitrogen-containing heterocycles are key structural motifs in bioactive molecules and materials, which could be synthesized by the intramolecular cyclization of nitrogen-centered radicals. In this study, the cyclization selectivity of nitrogen-centered radical reactions in ene-amide substrates initiated by proton-coupled electron transfer (PCET) under photoredox catalytic conditions is investigated. Contrary to prior knowledge that the 5-exo-trig/6-endo-trig cyclization selectivity could be switched by a phenyl tether, our experimental and computational analyses demonstrate that 5-exo-trig cyclization dominates, yielding γ-lactams as major products, while 6-endo-trig pathways are disfavored unless specific substituents (e.g., α-phenyl or sulfonamide groups) modulate reactivity. These findings complement the established conceptions of radical cyclization of ene-amide substrates and offer novel strategies for synthesizing functionalized lactams.