Complementary Site Selectivity in Ortho-Alkylative Vicinal Difunctionalization Reactions of Iodoarenes Enabled by Palladium–Olefin Catalysis.
Abstract
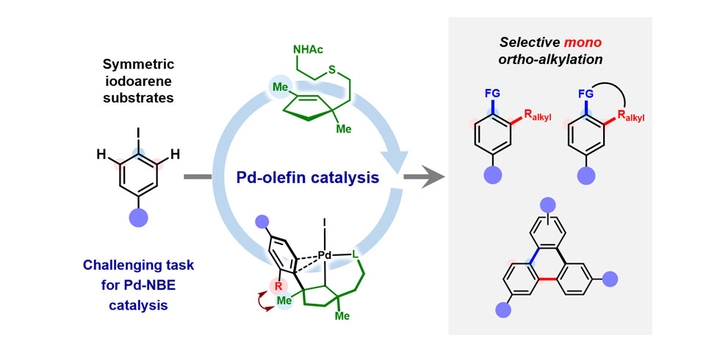
Direct access to multifunctionalized arenes through regioselective vicinal difunctionalization of aryl substrates is a challenging yet highly sought-after process. Palladium–norbornene catalysis enables the synthesis of ipso,ortho-difunctionalized products from haloarenes; however, selective mono-ortho-alkylative vicinal difunctionalization reactions of para-substituted haloarene substrates have remained elusive. Here we report the use of a methyl-modified thio-cycloolefin ligand for the palladium-catalysed ortho-alkylative vicinal difunctionalization of para-substituted iodoarenes. This catalytic system demonstrates compatibility with a variety of para-substituted iodoarenes, alkyl iodides and termination reagents, facilitating ortho-alkylative vicinal difunctionalization unattainable by conventional palladium–norbornene catalysis. In addition, the catalytic system can be used for the selective mono-ortho-arylation of para-substituted iodoarenes, enabling the synthesis of triphenylenes. Mechanistic studies reveal the origins of site selectivity within the catalytic process, through isolation of key intermediates and examination of their stoichiometric reactivity. This work highlights the versatility of palladium–olefin catalysis in addressing the complexities associated with constructing multifunctionalized aromatic frameworks through rational molecular design.
Highlights
- Highlighted in Synfacts 2026, 22, 154.
- Promoted by 科学网.