New Insights into the Torquoselectivity of the Staudinger Reaction.
Abstract
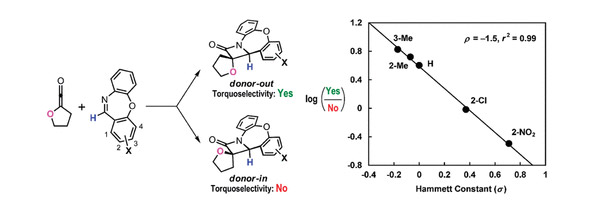
To understand the torquoselectivity and the electronic effects of the Staudinger reaction, a study using a combination of experiments and DFT calculations has been conducted for the reactions of an unsymmetric cyclic ketene and cyclic imines with different electronic properties. The predominant formation of the donor-in β-lactams, the torquoelectronically disfavored products, was observed for the first time. The Hammett analyses reveal a close relationship between the donor-out/donor-in product ratio and the electronic nature of the imines. The kinetic competition experiments and DFT calculations indicate that the two-step Staudinger reaction shows different rate-determining steps in the donor-in and donor-out pathways. Our investigations reveal that the torquoelectronic control in the Staudinger reaction is quite different from that in the ring-opening reaction of cyclobutene derivatives. The diastereoselectivity of the Staudinger reaction involving an unsymmetric disubstituted ketene cannot be simply rationalized or predicted by the torquoelectronic model.