Tandem Rh(I)-Catalyzed [(5+2)+1] Cycloaddition/Aldol Reaction for the Construction of Linear Triquinane Skeleton: Total Syntheses of (±)-Hirsutene and (±)-1-Desoxyhypnophilin.
Abstract
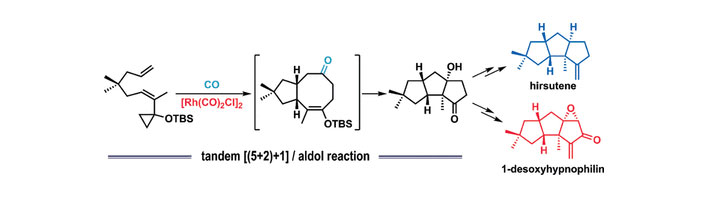
A tandem reaction involving a Rh(I)-catalyzed two-component [(5+2)+1] cycloaddition and an aldol condensation has been developed to construct the tricyclo[6.3.0.02,6]undecane skeleton and its heteroatom-imbedded analogues. Meanwhile, this method has been successfully applied to natural product synthesis for the first time. The present strategy enables a straightforward approach to the natural linear triquinane skeleton, as demonstrated by concise and step economical syntheses of hirsutene and 1-desoxy-hypnophilin, whereby the linear triquinane core is diastereoselectively established in one manipulation with correct placement of all stereocenters, including two quarternary centers. This first application of the Rh(I)-catalyzed [(5+2)+1] cycloaddition in natural product synthesis highlights the efficiency of this methodology for constructing complex fused ring systems.